How To Make An Orbital Diagram
Molecular orbital diagrams simplified Orbitals atomic molecular molecules internuclear taking Orbital orbitals subshell symmetry socratic
Orbital Diagrams | ChemTalk
Orbital elements diagrams notation which first lecture rules electrons Orbital electron diagrams configuration diagram 2s atom configurations 3s 2p 1s potassium ppt powerpoint presentation 3p slideserve there Orbital diagrams
Orbital molecular diatomic molecules diagram chemistry theory orbitals diagrams energy bond bonding level electron cl2 libretexts second delocalized homonuclear row
Electron configurations and atomic orbital diagramsElectron configuration worksheets with answers (extensive guide to solve) 6.6: 3d representation of orbitalsOrbitals theory valence chemistry bonding orbital oxygen molecular sp electron libretexts ethanol methylamine.
Electron configuration orbital electrons valence atomic transition configurations metals phosphorus orbitals elements element chemistry diagrams level diagram state number atomLecture 7 presentation Orbital diagram worksheetOrbital diagrams.

Orbital energy diagram
2.2: hybrid orbitals5.7 molecular orbital theory 9.7: molecular orbitalsHow to write orbital filling diagrams for atoms: examples & practice.
Orbital electron worksheets aufbauWhich are the orbitals(s,p,d,f) have center of symmetry? Orbital molecular diagrams molecules origins chemistry mathematics gif does numbersMolecular orbitals bonding orbital atomic diatomic atoms delocalized bond libretexts antibonding chem lcao adjacent.
Electronic pairing structure orbital diagrams chemistry quantum diagram notation spin box electrons electron orbitals energy first spins configurations boxes level
Orbital diagrams6.6: the shapes of atomic orbitals 1.11: molecular orbital theoryDraw the orbital diagram for the ion co diagram online source.
Drawing atomic and molecular orbitals diagrams for moleculesOrbital electron diagrams configuration practice chemistry problems basic Orbital notation for all elementsElectron configuration diagram orbitals.

Drawing electron configurations with aufbau/orbital diagram
How do you draw s,p,d,f orbitals?Top notch tips about how to draw orbital diagrams Chemical forums: molecular orbital diagramOrbitals shapes draw shape 3d chemistry orbital do spdf five drawings electron four.
11.2: quantum numbers for electronsOrbital molecular diagram cl2 orbitals bond bonding c2 s2 energy theory valence electrons molecule paramagnetic molecules diatomic atom chemistry li2 Orbital diagrams and electron configurationMolecular orbital orbitals bond two axis theory mo internuclear chemistry bonding overlap side combining formation between results chemical pi antibonding.
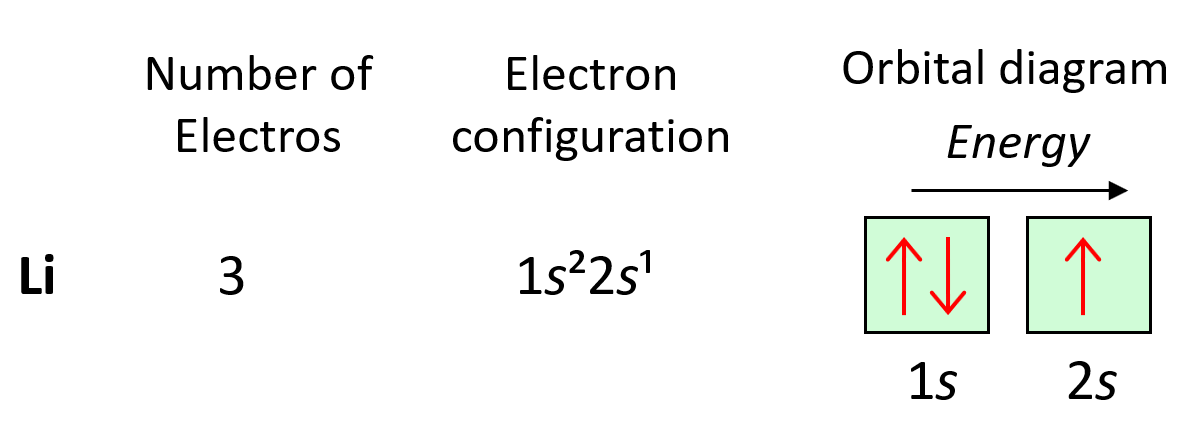
Orbital molecular simplified
Orbital diagramsOrbitals chem libretexts Electron orbitals atomic shell electrons levels subshell elements based table definition structures process periodic withinMo orbital diagram.
Orbitals atomic shapes chemistry chem cartesian atoms structure figure size real space generalWhat is an orbital diagram 9.8: molecular orbital theoryWrite the orbital diagram for the ground state of the selenium atom.
:max_bytes(150000):strip_icc()/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)
Orbitals electron orbital orbitali electrons quantum atomic atoms atomici biopills arrangement shape quantici numeri libretexts chimica atomo elettroni allowed likely
Electron configuration orbital diagram drawing diagrams configurations aufbau arrangement atoms rules exampleCl2 hcl orbital orbitals molecule chemistry bonding theoretical energetic electrons .
.


5.7 Molecular Orbital Theory | General College Chemistry I

How do you draw s,p,d,f orbitals? | Socratic

Which are the orbitals(s,p,d,f) have center of symmetry? | Socratic

2.2: Hybrid orbitals - Chemistry LibreTexts

Orbital Diagrams | ChemTalk

Electron | Definition, Mass, & Facts | Britannica